| ISBER 2024 Regional Meeting - St. Petersburg, Florida |
ISBER 2024 Regional MeetingThe ISBER 2024 Regional Meeting will be held in St. Petersburg from November 5-6, 2024. Year after year, ISBER brings together new and returning biobankers from around the world, providing opportunities for academia, industry, and pharma to learn, connect, discuss, and collaborate. **In light of the recent impacts from hurricanes Milton and Helene, we want to assure you that we are closely monitoring the situation and prioritizing the safety and well-being of all attendees. We have heard from our venue and our program planning task force members based in the St. Petersburg/Tampa-area that they expect to be able to welcome delegates to the ISBER Regional Meeting on Nov. 5-6. Our event is scheduled to proceed as planned. We are in communication with local partners to ensure everything is in place for a safe and successful meeting. We look forward to welcoming you to St Petersburg, FL.**  ISBER 2024 Regional MEETINGSt. PETERSBURG, FLORIDA, USA | NOVEMBER 5-6, 2024
Registration
ISBER 2024 Regional Meeting
ISBER is excited to announce its 2024 Regional Conference in St. Petersburg, Florida. This meeting will address topics that showcase the benefits of biobanking networks for sample procurement, usage and sharing. Biobanking networks will impact collaborations in human, environmental and microbial collections and enhance diversity, public good and precision medicine. Early applications of AI technologies to increase security and traceability of biobank samples will be discussed, together with quality and sustainability matters. Come join us in sunny Florida as we promote discussions for a brighter future for biobanking. Meeting theme: "Biobanking for a Brighter Tomorrow" IMPORTANT INFORMATION
*If you are not a current ISBER student/technician member, please submit proof of your student status or a letter from your employer to info@isber.org to receive the student/technician rate. Low and Middle Income Countries (LMICs): Delegates in countries identified by the World Bank as Low and Lower-Middle Income are eligible for a 50% discount. Delegates in countries identified as Upper-Middle Income are eligible for a 25% discount. If you are eligible to receive a discount please contact info@isber.org. To confirm the income classification for your country, please click here. Please note that if you are also a technician or student, you will receive only the LMIC discount or the technician/student discount, whichever is higher.
All times are listed in local time (St. Petersburg, Florida). "...In the exhibit hall we could meet different vendors, service providers and brands, which is helpful to have an overview of new products and trends. I could also meet other colleagues from all over the world and exchange ideas, challenges... In general I found the ISBER meeting a very positive experience." 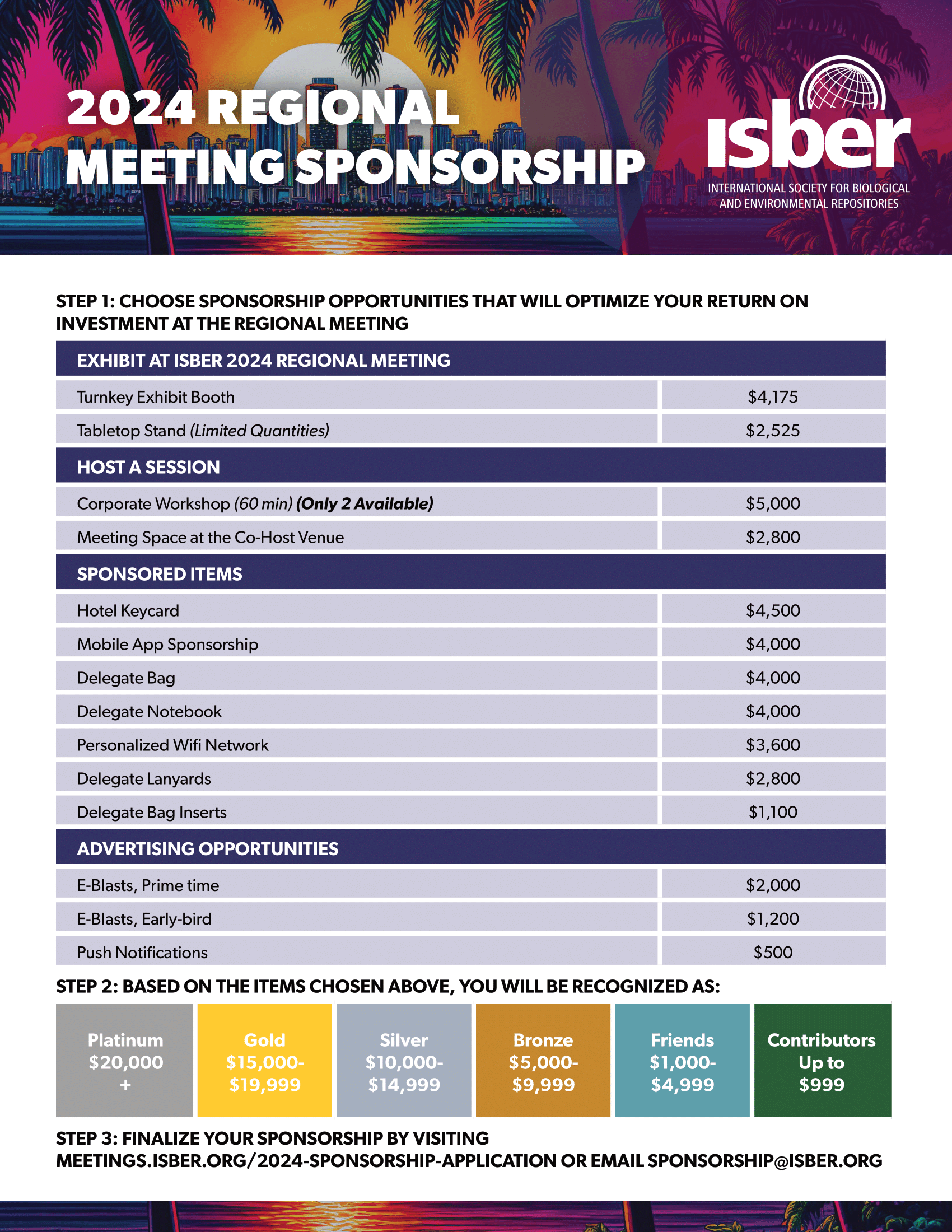 Check out the Corporate Opportunities here
Check out the Corporate Opportunities hereISBER would like to express gratitude and acknowledge the following companies and organizations for their generous support at the 2024 Regional MeetingAzenta Life SciencesAzenta life sciences is dedicated to enabling life sciences organizations around the world to bring impactful breakthroughs and therapies to market – faster. Azenta
encapsulates our commitment to helping customers reach new heights in their pursuit of scientific progress. By integrating our industry-leading capabilities, Azenta
Life Sciences enterprise-wide sample exploration and management solutions will accelerate discovery, development and delivery, with greater speed and precision.
Azenta strives to keep elevating each other, their customers’ work and industry – building a healthier world for people everywhere.
Brady CorporationBSI SystemsBSI Systems (BSI, BioShare, and SRL Advantage) is a collection of specimen inventory and resource management products that provide workflow, inventory, and location
tracking services for your facility. BSI manages your biobank with a validated software that tracks the complete life cycle of specimens within your repository.
BioShare is a web platform for sharing specimens and datasets across a research community. SRL Advantage can aid researchers to locate specimens across a biobanking
network.
FARRAR - Trane TechnologiesFreezerworksConfigurable software solutions for biological sample and study management. Track sample data across multiple freezers while managing workflow. Flexible and user-friendly,
Freezerworks puts the laboratory in control with easy-to-build fields, screens, and reports. Safeguard data with comprehensive security features, 21 CFR part 11
compliance, and cryogenic-safe barcode labeling. GA InternationalGA International has over 25 years of experience as a leading manufacturer of specialty labels, supplying laboratory identification solutions to biomedical research
labs, hospitals, and other healthcare institutions. Since its inception, GA International has become a worldwide leader in cryogenic and chemical-resistant labels,
with a strong dedication to R&D and customer service. Hamilton StorageHamilton Storage is a global leader in the design and manufacture of automated storage systems for biological and compound samples. Our solutions safeguard sample
integrity, empower researchers, and improve laboratory efficiency. We are committed to developing innovative technologies to meet the needs of the life science
industry.
IC BiomedicalCapitalizing on a 67-year legacy of cold chain storage and transport technology, IC Biomedical builds the highest-quality cryogenic storage and transport systems for the global biomedical research and development, healthcare, biorepository, pharmaceutical, biotechnology, IVF and animal husbandry markets. Our next-generation Revolution Series of high-capacity freezers sets the standard for high sample security, regulatory compliance and low operational costs. Manufactured at our medical-grade, ISO 13485-certified facility in Cartersville, Georgia USA. Kaye - Subsidiary of AmphenolFor more than 65 years, Kaye has been at the forefront of high accuracy process measurement. For applications from thermal process validation and environmental monitoring
to sensor calibration, Kaye technology has provided the most accurate and user-friendly measuring systems available in the market today. Kaye equipment has become
the standard for helping customers increase validation process efficiency and document the results. LiconicManifold As the amount of biomedical data types and scale continues to grow, old ways of working with data hold back the pace of progress — fragmented data, overwhelming omics,
complex manual work, analysis backlogs, friction in secure collaboration, and barriers to distributing workflows. Manifold is a health research infrastructure
company that enables researchers to focus on the high-impact research that matters most, by taking care of all the other stuff that gets in the way. Modul-BioModul-Bio specialises in computer solutions for biological sample management, implementing barcode systems, Biobank Information Management Systems (BIMS) and collaborative
tools for sharing biological sample collections. Over 300 laboratories around the world utilise our services. MBioLIMS Biobanking Software is a specifically designed
tool helping biobanks, biorepositories and cohort studies manage their biological and clinical data. OriginCellOriginCell specializes in cutting edge R&D and manufacturing of intelligent and fully automatic cryogenic biobanking systems. Our highly skilled team consists
of a diverse group of scientists and engineers from various disciplines. With exceptional expertise in cryopreservation and automation technologies, OriginCell
offers comprehensive and one stop solution of intelligent biostorage systems. Testo North AmericaTesto is a world leader in environmental monitoring, data analysis products and GxP services. Our focus on 21 CFR Part 11 Compliant Environmental Monitoring allows
our customers to know that when an audit happens, we have them covered. You never miss a monitoring point with Testo’s intuitive Saveris 1 software. Backed by
over 60 years of measuring engineering experience, our mission is to provide the best quality, service, and value in the industry. Zero Gravity ZERO GRAVITY SKIN was founded with one goal in mind; to develop and deliver the safest and most effective pain management and anti-aging Medical devices to consumers
across the globe. Zero Gravity is committed to produce the most effective LED (Light Emitting Diodes) light therapy for chronic pain and facial skin rejuvenation
in the industry. Hilton St. Petersburg Carillon Park We have a room block reserved at the Hilton St. Petersburg Carillon Park for November 4, 2024 through November 7, 2024. Booking your room is simple: select "Book a Room HERE" to receive your group's preferred rate. You may also call Hilton reservations at 1-800-774-1500 and mention your group code, "ISBER1." Space is limited and available first-come-first-serve. Please book before the deadline to take advantage of these room rates.
ROOM RATE DEADLINE: Task Force Responsibilities:
Task Force Co-Chairs:  Edward Seijo, MS Edward Seijo, MSH. Lee Moffitt Cancer Center & Research Institute Florida, USA Ed has been at Moffitt since 1999 and served in a wide variety of increasing technical and leadership roles, ranging from the establishing the Center’s Analytic Microscopy Core, followed by expansion and oversight of Moffitt’s Tissue Core, from which he transitioned to a more overarching role as Director, Shared Resources, to his current role of Senior Director. He is responsible for the oversight, strategic planning, and operations of all translational science shared resources, including but not limited to the Cell Therapies Core, Immune Monitoring Core, Tissue Core, PK/PD Core and Clinical Trials Laboratory Core. He serves as an institutional resource related to biospecimen governance, biobanking best practices, and co-author of the Center’s biospecimen policy. He has served on Moffitt’s biospecimen focused Scientific Review Committee for over 14 years and is an active College of American Pathologists Biorepository Inspector. In addition, he frequently serves as the Research Business Owner for a wide variety of Research Initiatives and developing supporting business plans, ranging from Cell Therapies Core Expansion, to current in-flight projects like Moffitt’s new Gene Engineering Facility, opening in late June 2023 and the new Speros Research Building, scheduled to open in early 2026.  Stella Somiari, PhD, MBA Stella Somiari, PhD, MBA
Windber Research Institute (WRI) Pennsylvania, USA Dr. Somiari is a Senior Director at the Windber Research Institute (WRI) and she directs the institute’s comprehensive biobank. She also conducts cancer and biospecimen science research using genomics and proteomics technologies. Dr. Somiari led the conceptualization, design and setting up of the fully integrated and comprehensive WRI biobank in 2000. The biobank is accredited by the College of American Pathologists (CAP) and its services include sample management, processing and distribution. The biobank serves WRI, and is the centralized biobank for a) the Department of Defense (DoD) funded Clinical Breast Care Project (CBCP) at Walter Reed National Military Medical Center (WRNMMC) which contributed breast tissue to The Cancer Genome Atlas Project (TCGA), b) the DoD’s Murtha Cancer Research Program (MCCRP) of WRNMMC/Uniformed Services University (USU), a network of 9 Military Treatment Facilities and Medical Centers, and 7 Veteran Affairs (VA) Medical Centers, c) the DoD Center for Prostate Disease Research (CPDR), c) The Applied Proteogenomic Organizational Learning and Outcomes (APPOLLO) network, a collaboration between NCI, DoD and the Department of Veteran Affairs and d) the recently established PROject for Military Exposures and Toxin History Evaluation in US service members (PROMETHEUS). Dr. Somiari has been an active member of ISBER for over 19 years and has served on several committees including conference planning committees (Annual and Regional) and the Task Force on ISO-Biotechnology-Biobanking General Requirements. She has been Associate Editor/contributor to three ISBER Best Practices publications. She consults as a subject matter specialist on biobanking matters. Task Force Members:
Bushra Rakha
Marianne Henderson
Anna Michalska-Falkowska
Rocio Aguilar-Quesada
Judith Giri
Rania Salem
Vanessa Tumilasci
Gouri Mahajan
Billy Schleif
Lori Campbell
Quynh Vantu
David Carpentieri
Jennifer Ness
Judita Kinkorova Planning your visit to the St. Petersburg/Clearwater area? Click the links below to find more information about things to do in the area as well as access the Destination Guide from the St. Petersburg/Clearwater tourism board.
|
8/27/2025 » 12/31/2025
ISBER Best Practice Webinar Series - Full 5-Webinar Series
2/3/2026 » 2/5/2026
Biobanking 101 Workshop: Planning and Operational Setup: Planning and Managing Storage Environm
4/21/2026 » 4/23/2026
ISBER 2026 Global Biobanking Congress in Shenzhen, China